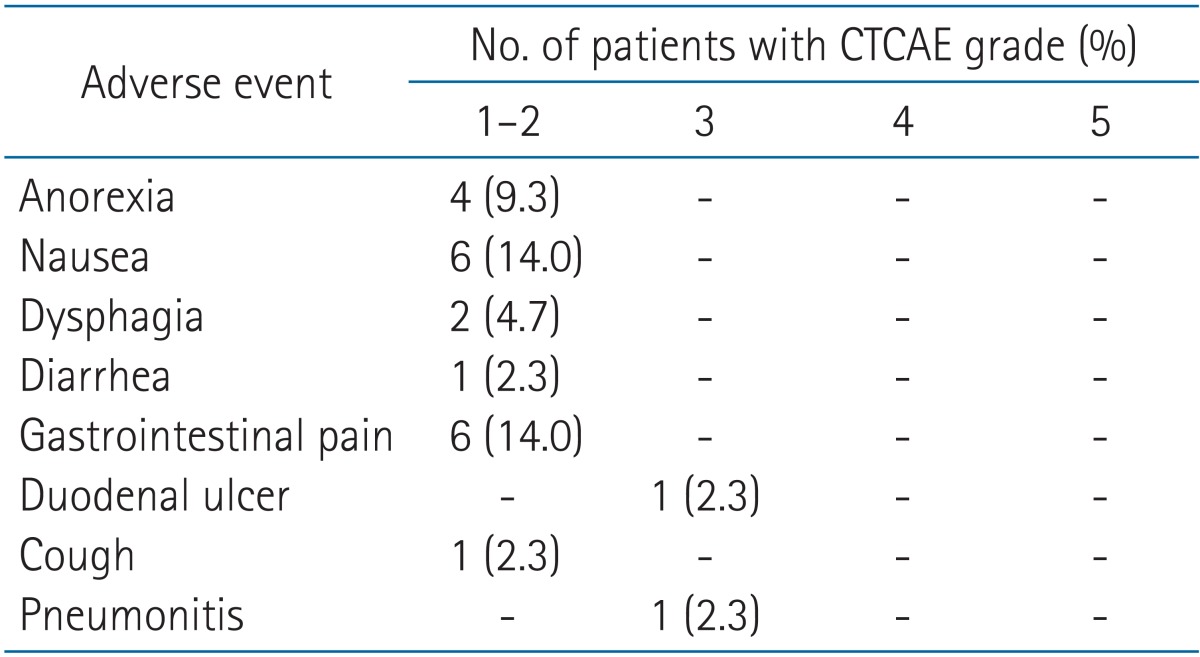
![PDF] Patient-reported outcomes and the evolution of adverse event reporting in oncology. | Semantic Scholar PDF] Patient-reported outcomes and the evolution of adverse event reporting in oncology. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/72d6066aa43136e48ae6226e3dba7e542a7a02de/5-Table1-1.png)
PDF] Patient-reported outcomes and the evolution of adverse event reporting in oncology. | Semantic Scholar

A review of criteria strictness in “Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials” - ScienceDirect

Book 3D: 2023 Common Terminology Criteria for Adverse Events (CTCAE) – Clinical Research Resources, LLC

Toxicity grading scale in the common terminology criteria for adverse... | Download Scientific Diagram

Composite grading algorithm for the National Cancer Institute's Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) - Ethan Basch, Claus Becker, Lauren J Rogak, Deborah Schrag, Bryce B





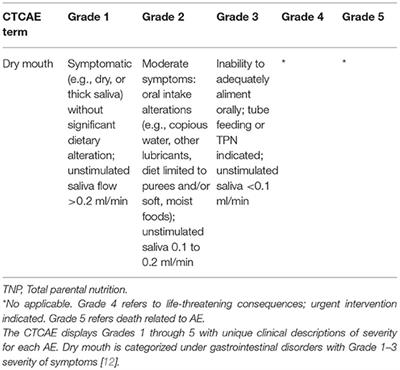
![Ctcae 4.02 2009-09-15_quickreference_8.5x11[1] | PDF Ctcae 4.02 2009-09-15_quickreference_8.5x11[1] | PDF](https://image.slidesharecdn.com/ctcae4-022009-09-15quickreference8-5x111-120711021618-phpapp01/85/ctcae-402-20090915quickreference85x111-3-320.jpg?cb=1668437655)